Rapamycin
The most powerful tool to stop the acceleration of aging caused by mTOR dysfunction and cellular senescence.
Hypertrophic cardiomyopathy (HCM)—a condition marked by thickening of the heart muscle and impaired relaxation—is a leading cause of heart failure in aging populations, yet remains largely untreatable at its root. Emerging research suggests that its underlying biology is driven not by passive wear-and-tear, but by overactive growth signaling pathways, particularly mTORC1. This article explores the hyperfunction theory of aging, pioneered by Dr. Mikhail Blagosklonny, which reframes age-related disease as a consequence of continued cellular overactivity beyond developmental need. We focus on the translational insights offered by domestic cats, one of the only species to naturally develop HCM in ways that mirror human disease. Early studies using low-dose rapamycin—a selective mTORC1 inhibitor—demonstrate promising cardiac remodeling, reduced fibrosis, and improved function in aging feline hearts. Together, these findings reveal not only a novel therapeutic pathway, but a broader framework for understanding and targeting the cellular biology of cardiac aging across species.
rapamycin
24 mins
By: Shreshtha Jolly
The heart is a muscular organ that beats nearly 100,000 times each day to circulate blood and sustain life. Unlike regenerative tissues such as the liver or skin, the heart has a limited ability to repair itself, making it particularly susceptible to the effects of aging. Over time, structural changes begin to occur: the walls of the heart thicken through a process known as hypertrophy, and its elasticity declines. As a result, blood flow becomes less efficient, often leading to exercise intolerance and reduced oxygen delivery throughout the body.
Beneath these physiological changes lies a subtle but persistent shift in molecular signaling—one that increasingly favors cellular growth over maintenance. At the center of this imbalance is mTOR (the mechanistic target of rapamycin), a key regulatory pathway that governs cellular metabolism, protein synthesis, and repair. In early life, mTOR activity supports rapid growth and development, enabling cells to build, divide, and respond to energy demands. But when persistently active in adulthood, mTOR can drive pathological growth and impair the cell’s ability to clear damaged components. As mTOR activity increases, autophagy slows, cellular waste accumulates, and tissues like the heart become more vulnerable to dysfunction.
While much of our understanding of cardiac aging comes from human studies, similar patterns are observed across the animal kingdom. Aging is not a uniquely human phenomenon; many species experience parallel forms of functional decline, including in the cardiovascular system. Studying age-related disease in animals does more than serve as a model for human health; it can also highlight the unmet needs of aging animals themselves. Companion animals, like dogs and cats, are increasingly living into old age, and they too face chronic conditions that compromise quality of life. One of the most striking examples is hypertrophic cardiomyopathy (HCM) in domestic cats.
Feline HCM is the most common heart disease in cats, characterized by abnormal thickening of the heart walls that reduces its ability to fill and pump efficiently. Unlike in lab-engineered models, this condition arises spontaneously in cats, closely resembling the natural progression of human heart failure. The parallels are not just anatomical but molecular, suggesting that feline cardiac aging may offer a highly translational model for understanding shared pathways, including the role of mTOR signaling.
This is where rapamycin becomes particularly compelling. By selectively inhibiting mTOR, rapamycin promotes autophagy, reduces cellular stress, and helps restore a healthier balance between growth and repair. In preclinical studies, it has extended lifespan and improved cardiac function in aging mice. Early investigations in cats suggest that rapamycin may also reverse or slow the progression of HCM, offering potential benefits not just for translational science, but for the health of the animals themselves.
Recently, building on this emerging body of evidence, Petspan—a veterinary telemedicine platform developed by Healthspan to address the cellular drivers of aging in companion animals—launched a clinical program delivering rapamycin to cats diagnosed with HCM. By combining targeted dosing, specialist oversight, and ongoing monitoring, the protocol aims to make mTOR-modulating therapies accessible to aging cats living with HCM
In this review, we examine the role of mTOR signaling in cardiac aging and hypertrophic cardiomyopathy, with a particular focus on the translational value of feline models. We explore emerging evidence on the therapeutic potential of rapamycin across species, and consider how these findings might inform future interventions for age-related cardiac dysfunction in humans (and animal companions).
One of the key players in the aging process is a protein called mTOR. It works like a cellular growth control center, helping the cell respond to the amount of nutrients, energy, and growth signals it receives. When the growth-promoting form of mTOR, known as mTORC1, is active, it tells the cell to start building. This includes making more proteins, increasing in size, and preparing to divide. These actions are helpful during early growth and development. However, as we age, this constant push for growth can become harmful. At that stage of life, the body needs to focus more on repair and maintenance rather than continued expansion [1, 2].
As we grow older, mTORC1 often stays active even when the body no longer needs to grow. This constant activity becomes harmful because it blocks a crucial cellular process called autophagy. Autophagy is like the cell’s cleanup system, it removes and recycles damaged parts, such as worn-out mitochondria and misfolded proteins. Mitochondria refer to the powerhouses of our cells. They help generate energy in the form of adenosine triphosphate (ATP), which is the energy currency of the body.
Unfortunately, when worn-out mitochondria and other organelles build up inside the cell due to disruptions in autophagy, oxidative stress increases, energy production declines, and the cell’s ability to function properly starts to break down.
This kind of imbalance is especially damaging to the heart. Heart muscle cells, known as cardiac myocytes, don’t divide often. That means they can’t easily replace themselves or remove waste. Over time, damaged mitochondria and other cellular debris build up. To compensate, the cells begin to grow in size, but not in a healthy way. This triggers a condition known as cardiac hypertrophy, where the walls of the heart become thicker and the chambers inside shrink. A thicker heart wall may sound strong, but it actually makes it harder for the heart to fill with blood and pump it efficiently.
In the early stages, this thickening might seem helpful. The heart can push harder against resistance, generating more force. But this advantage doesn’t last. As the walls thicken, the heart becomes stiffer and loses its ability to stretch. It struggles to fill between beats, causing blood to back up into the lungs or other parts of the body. This results in a condition called Heart Failure with Preserved Ejection Fraction, or HFpEF. In HFpEF, the heart’s pumping strength appears normal, but it can’t relax properly between beats. That means less blood is taken in, and less is sent out with each heartbeat [3].
At the same time, another related condition can develop: Hypertrophic Cardiomyopathy (HCM). While HCM is often thought of as a genetic disease seen in young athletes, recent research shows that a non-genetic, age-related form also exists. It develops slowly due to long-term stress on the heart, mTOR overactivity, and impaired autophagy. These changes lead to thickening of the heart walls and the development of fibrosis, a buildup of scar-like tissue made of collagen and other structural proteins.
Fibrosis acts like glue inside the heart. As fibrous tissue builds up, it causes the heart muscle to become stiff and less flexible. This stiffness makes it harder for the heart to stretch and contract properly with each beat, reducing its ability to fill with blood and pump it out efficiently. Fibrosis also disrupts the normal electrical signals that regulate the heartbeat. This disruption can cause irregular heartbeats, known as arrhythmias. In severe cases, these irregular rhythms can lead to sudden cardiac arrest or heart failure.
Adding to the problem, fibrosis places extra strain on the heart’s energy system. The mitochondria, which are the powerhouses inside the heart cells, have to work harder to meet the increased energy demands. However, in aging cells that are already under stress, this additional burden causes further energy loss and more cellular damage. The result is a heart that becomes weaker and less able to function over time.
When hypertrophic cardiomyopathy, or HCM, begins to manifest, it can range from subtle symptoms to more serious cardiovascular events. In some individuals, it leads to congestive heart failure, where thickened heart walls impair the heart’s ability to relax and fill properly. This can cause blood to back up into the lungs or lower body, resulting in fatigue, shortness of breath, and swelling in the legs or abdomen, often during activities that previously felt easy.
In other cases, HCM may lead to the formation of blood clots, which can travel through the bloodstream and block critical arteries. One example is aortic saddle thromboembolism, which can suddenly impair blood flow to the legs, causing pain, numbness, or coldness that may require urgent care. In rare but severe instances, disruptions in the heart’s electrical system can lead to irregular rhythms like ventricular fibrillation, which can be life-threatening without immediate treatment.
Such sudden manifestation of HCM, whether as congestive heart failure, aortic saddle thromboembolism, or sudden arrhythmic death, is not unique to humans. It is also seen in our animal companions, particularly in cats. Domestic cats are one of the few species that naturally develop hypertrophic cardiomyopathy without genetic modification or experimental induction. This makes them a particularly valuable model for studying cardiac aging and disease progression.
One of the most comprehensive efforts to understand feline HCM is the REVEAL registry, an international observational study that followed more than 1,700 pet cats. These included cats with early-stage HCM, with and without obstruction to blood flow, as well as healthy controls. The registry tracked how HCM develops and progresses over time, including the frequency and severity of adverse cardiac events [4].
The findings were sobering. Nearly one in three cats with HCM went on to develop major heart complications, such as congestive heart failure or thromboembolism—blood clots that can block blood flow to the limbs, often resulting in sudden paralysis or death. Approximately 28% of cats died from heart-related causes. Once clinical symptoms emerged, median survival time was typically only about one additional year. These outcomes highlight how feline HCM can remain silent for long periods before rapidly becoming life-threatening.
Research like this not only improves veterinary care for aging animals, it also informs human medicine. Because the molecular and physiological mechanisms of HCM appear similar across species, studying its progression in cats offers a unique translational opportunity. Insights gained from feline models can deepen our understanding of how cardiac aging unfolds in humans, and how it might be prevented or slowed.
The cardiac remodeling observed in hypertrophic cardiomyopathy (HCM) doesn’t occur in isolation—it is part of a broader biological pattern seen in aging across organ systems. With age, a convergence of factors such as chronic low-grade inflammation (inflammaging), mitochondrial inefficiency, insulin resistance, and vascular stiffening creates an environment in which the heart is under constant pressure to adapt. At the molecular level, one of the central conductors of this maladaptive symphony is mTORC1—a nutrient- and growth-sensitive complex that promotes cellular biosynthesis, proliferation, and anabolic metabolism.
In early life, mTORC1 plays a vital role in orchestrating development, helping cells grow, divide, and specialize. But as longevity researcher Dr. Mikhail Blagosklonny argues in his hyperfunction theory of aging, problems arise when this growth program continues unchecked into adulthood. Rather than viewing aging as a passive decline from wear and tear, the hyperfunction theory frames it as a case of biological run-on sentences—where the machinery of growth and adaptation fails to shut off, even when it begins doing more harm than good.
In this model, aging is driven by the continuation of growth programs beyond their useful window (during childhood development), leading to excessive cell proliferation (hyperplasia), cell enlargement (hypertrophy), and dysfunctional cellular overactivity (hyperfunction) later in life [5]. In the heart, HCM provides a vivid example. Cardiomyocytes—the muscle cells of the heart—undergo pathological hypertrophy, not because they are failing, but because they are over-functioning. These cells enlarge in response to continued anabolic signaling, especially from the mTORC1 pathway, even when such growth is no longer appropriate. The result is a thickened, stiff heart wall that impairs diastolic filling, increases oxygen demand, and ultimately limits cardiac performance.
Rapamycin addresses this problem at its source. By selectively inhibiting mTORC1, rapamycin shifts the cellular state from growth to maintenance. It reactivates autophagy—the cell’s internal recycling system—thereby helping clear dysfunctional mitochondria, reduce oxidative stress, and improve metabolic efficiency. In effect, rapamycin doesn’t just suppress downstream symptoms; it recalibrates the cell’s core operating system.
This pharmacologic “off switch” to the hyperfunction program has shown encouraging results in animal studies, including naturally aging cats. In these models, rapamycin administration has been associated with reduced myocardial wall thickness, less interstitial fibrosis, and improved ventricular relaxation—hallmarks of restored structural and functional reserve in the aging heart. These findings underscore a powerful therapeutic principle: by dialing down overactive growth signals, we may not only delay the progression of HCM, but potentially reverse some of its pathological features [6, 7].
HCM is the most common form of heart disease in felines, characterized by thickening of the left ventricular wall that reduces the heart’s ability to relax and fill properly. What makes this condition particularly significant is that it closely mirrors human heart failure with preserved ejection fraction (HFpEF) and age-related, non-genetic HCM, both in its natural course and in its underlying molecular features.
In a landmark observational study known as the CatScan Study, researchers examined 1,007 cats over the age of six months [8]. All of the cats were healthy at the time of enrollment and were available for rehoming from two animal shelters during a 17-month study period. To isolate true age-related HCM, the researchers excluded cats with high blood pressure (hypertension) or hyperthyroidism—two conditions known to affect heart structure.
Each cat underwent a thorough cardiac evaluation, including heart auscultation, blood pressure measurement, and two-dimensional echocardiography. This ultrasound-based imaging allowed researchers to measure the thickness of the heart walls. A diagnosis of HCM was made if the left ventricular wall measured 6 millimeters or more during diastole, the heart’s relaxed phase. Among the 780 cats with complete data, nearly 15% met criteria for HCM. By comparison, only 0.5% had congenital heart defects, and just 0.1% had other forms of cardiomyopathy, making HCM the predominant form of heart disease observed.
Certain breeds such as Maine Coons and Ragdolls are known to carry mutations in the MYBPC3 gene, which encodes for a protein critical to the structure and contractility of cardiac muscle. Mutations in this gene disrupt normal protein function, leading to thickening of the heart wall over time. However, many of the affected cats in the CatScan study were mixed-breed animals with no known genetic predisposition. This suggests that age-related HCM, driven by environmental and metabolic stressors, including chronic mTOR activation, plays a substantial role. Unlike genetic forms of the disease that appear earlier in life, this variant progresses gradually, with wall thickening and diastolic dysfunction emerging over years.
What makes cats uniquely valuable in aging research is not just the spontaneous development of HCM, but the striking resemblance of their disease trajectory, molecular biology, and treatment responses to those seen in humans. Unlike rodent models, which often rely on surgical or genetic induction of disease, feline HCM occurs naturally, offering a more accurate representation of how heart disease unfolds in real-world aging.
At the molecular level, feline HCM shows many of the same hallmarks found in aging human hearts. These include increased mTORC1 activity, impaired autophagy, mitochondrial dysfunction, and excess collagen deposition, also known as fibrosis. Postmortem analysis of feline heart tissue has revealed elevated levels of S6K1 and 4EBP1, downstream effectors of mTORC1 signaling that indicate heightened anabolic drive and cellular stress. In parallel, the accumulation of collagen between cardiac muscle cells further confirms a remodeling process that limits flexibility and interferes with normal electrical conduction [9].
Another practical advantage of studying feline HCM is the compressed timescale. While humans may develop cardiac disease over the course of decades, cats tend to exhibit the full clinical arc of HCM, including early hypertrophy, reduced diastolic function, arrhythmias, and eventual heart failure, within 5 to 10 years. This allows researchers to observe both the natural course and the impact of interventions like rapamycin over a manageable study period.
Moreover, cats do not live in controlled lab environments. They are exposed to dietary variability, stress, pollutants, and sedentary behaviors, just like humans. This ecological validity enhances the translatability of feline data to human clinical care. When a treatment shows efficacy in naturally aging cats living in typical home environments, it increases the likelihood that similar effects could be seen in human patients.
This unique convergence, shared molecular mechanisms, spontaneous disease progression, and real-world exposure, makes feline HCM an incredibly valuable model for studying cardiac aging. It offers not only insights into the biology of heart failure, but also an opportunity to test therapies that may benefit both humans and companion animals alike. As we’ll explore next, rapamycin is showing promising results in exactly this setting.
By suppressing mTORC1 activity, rapamycin reawakens the cell’s autophagic machinery—the system responsible for clearing damaged proteins and organelles—and reduces the kind of maladaptive tissue remodeling seen in conditions like HCM. This shift from growth to maintenance offers a promising strategy for restoring balance in aging tissues. But as with many powerful interventions, dose and duration matter.
Early studies found that daily, high-dose rapamycin, while effective at dampening mTORC1, could also interfere with mTORC2—a closely related complex with a very different role. While mTORC1 drives growth and protein synthesis, mTORC2 is essential for maintaining both insulin sensitivity and proper immune function. It helps regulate the activity and survival of immune cells, particularly T cells and dendritic cells, and is involved in signaling pathways critical to mounting an effective immune response.
Disrupting mTORC2 can therefore impair insulin signaling—leading to glucose intolerance and other hallmarks of metabolic syndrome—and at the same time, weaken immune competency, increasing susceptibility to infections and reducing vaccine responsiveness. In this context, chronic mTORC2 inhibition may mirror the immunosuppressive profile observed in transplant patients, where rapamycin is used deliberately to prevent organ rejection by suppressing T cell proliferation.
A 2012 study led by Dudley Lamming showed that chronic rapamycin use impaired insulin signaling in mice, raising concerns about glucose intolerance and potential long-term side effects [10]. In parallel, high doses over extended periods also dampened immune cell function, consistent with rapamycin’s origin as an immunosuppressant in organ transplantation. In this context, rapamycin blunts the proliferation and activation of T cells, which—while beneficial in preventing graft rejection—raises concerns when aiming to preserve immune competence in otherwise healthy individuals.
In response, researchers began exploring intermittent dosing strategies, such as once-weekly administration, to target mTORC1 without significantly affecting mTORC2. This approach preserves the cellular benefits of rapamycin (such as improved autophagy and reduced hypertrophy) while minimizing metabolic disruption. It was this refined strategy that inspired researchers to test rapamycin in a real-world setting, using a model with both translational and clinical relevance: domestic cats with naturally occurring HCM.
In a compelling pilot study conducted by Rivas et al. (2023), researchers evaluated whether once-weekly rapamycin could safely improve cardiac health in cats diagnosed with non-obstructive HCM [11].
The study enrolled 12 cats, of which nine received rapamycin by mouth once per week for eight weeks. Dosing was divided into two groups: a lower dose (0.025 mg/kg) and a higher dose (0.05 mg/kg). The remaining three cats served as untreated controls. Throughout the study, the animals underwent comprehensive assessments, including echocardiograms, electrocardiograms, blood and urine panels, to monitor heart structure, rhythm, inflammation, and organ function.
At the end of the study period, researchers performed detailed tissue analyses to examine changes at the molecular and cellular level. The results were striking.
Cats that received rapamycin showed gene expression changes suggestive of cardiac remodeling and improved cellular housekeeping. Genes involved in autophagy were upregulated, while those associated with pathological hypertrophy were suppressed. These changes were most prominent in the left ventricle and interventricular septum, two areas typically affected in feline and human HCM.
Three genes in particular, FBLN5, FREM1, and ITGB8, were consistently upregulated in treated cats. FBLN5 supports tissue elasticity and vascular integrity; FREM1 helps maintain structural cohesion under stress; and ITGB8 plays a role in resolving inflammation and promoting tissue repair. Collectively, these shifts point toward improved cardiac resilience and structural health.
Proteomic analyses (across blood, urine, and cardiac tissue) reinforced these findings. Treated cats showed lower levels of proteins linked to clot formation, inflammation, and cardiac stress, and higher levels of proteins associated with healthy metabolism, cytoskeletal organization, and protection from damage.
Crucially, the treatment was well tolerated. No major side effects were reported, and markers of kidney and liver function remained within normal ranges. This suggests that once-weekly rapamycin may be both safe and practical for use in a clinical or even at-home veterinary context.
This pilot trial represents the first controlled investigation demonstrating that low-dose, intermittent rapamycin can produce meaningful changes in cardiac gene expression and systemic health markers in cats with naturally occurring HCM. Just as importantly, it laid the foundation for a larger clinical trial, RAPACAT, which will expand on these findings and help determine whether these early signals translate into long-term improvements in feline heart function and survival.
The RAPACAT study represents the most compelling real-world test of whether rapamycin can intervene meaningfully in the progression of hypertrophic cardiomyopathy (HCM) in aging animals [12]. Conducted across two major veterinary centers, the trials were designed not as controlled lab experiments, but as clinical interventions in pet cats, living in home environments, cared for by families, and diagnosed with naturally occurring disease.
This distinction is important. Unlike genetically engineered mice or surgically induced disease models, the cats in this study developed HCM spontaneously, as humans often do. Their age, genetic background, lifestyle exposures, and disease trajectories reflected everyday conditions, giving the trial an unusually high level of translational relevance for human medicine.
The study enrolled middle-aged to older cats, typically between 6 and 13 years of age, who were diagnosed with early-stage HCM based on echocardiographic findings. These cats exhibited mild to moderate thickening of the left ventricular wall and impaired diastolic function, but preserved systolic ejection fraction, closely mimicking human HFpEF (heart failure with preserved ejection fraction). Cats were randomized into two groups: one received oral rapamycin (0.05 mg/kg, three times weekly), while the other received a placebo.
Over the 16-week treatment period, researchers conducted comprehensive cardiac and metabolic assessments. These included:
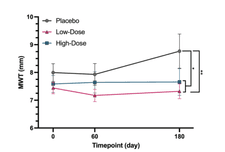
Structural Improvement in the Heart: Cats treated with rapamycin experienced an average 17–22% reduction in maximum left ventricular wall thickness (MWT), a key indicator of pathological hypertrophy. In contrast, the placebo group showed slight progression of wall thickening over time. This suggests that rapamycin not only stabilized the disease but may have reversed maladaptive cardiac remodeling.
Improved Diastolic Function: Treated cats showed measurable improvements in how efficiently the heart filled between beats. Importantly, this occurred without changes to ejection fraction, aligning with therapeutic targets in HFpEF: improving relaxation without compromising contractility.
Enhanced Cellular Repair Mechanisms: Molecular analyses revealed that rapamycin reactivated autophagy, with increased expression of autophagy-related proteins and restoration of autophagic flux. Concurrently, levels of S6K1 and 4EBP1, key markers of mTORC1 activity, were significantly reduced, confirming that the drug was engaging its intended target.
Reduced Fibrosis: Perhaps most strikingly, histological analysis showed marked reductions in myocardial fibrosis. Treated cats had fewer fibrotic regions in their heart tissue, suggesting that rapamycin may help halt or even reverse collagen accumulation. Since fibrosis contributes significantly to heart stiffness and arrhythmia risk, this finding represents a major therapeutic advance.
Favorable Safety Profile: Despite concerns about rapamycin’s immunosuppressive properties, no significant adverse events were reported. Liver enzymes, kidney markers, and glucose levels remained within normal limits. Treated cats did not develop infections, and none were withdrawn due to side effects.
Trajectory of maximum left ventricular wall thickness (MWT) over the 180-day study period in cats with HCM.
Figure 1. Trajectory of maximum left ventricular wall thickness (MWT) over the 180-day study period in cats with HCM. At baseline (Day 0), all groups, placebo (gray), low-dose rapamycin (pink), and high-dose rapamycin (teal), began with comparable MWT measurements, confirming balanced initial conditions. By Day 60, both treatment groups demonstrated a significant reduction in MWT, with this improvement sustained through Day 180. In contrast, the placebo group exhibited a gradual increase in wall thickness over time, reflecting the expected progression of disease. These findings indicate that once-weekly rapamycin may not only prevent further hypertrophy but actively reverse existing cardiac remodeling.
The RAPACAT findings offer strong preclinical evidence that rapamycin, when appropriately dosed, can safely reverse key features of age-related heart disease in a natural model. The study also validates the use of spontaneously aging animals over artificially manipulated models, reinforcing the translational value of veterinary cardiology for human health.
In the next section, we turn to the question this research inevitably raises: what does this mean for humans? Can we apply these findings to a population still searching for meaningful therapies to treat HFpEF, fibrosis, and the broader cardiovascular effects of aging?
Heart failure, particularly Heart Failure with Preserved Ejection Fraction (HFpEF), remains one of the most urgent and complex challenges in modern cardiology. It accounts for nearly half of all heart failure cases, disproportionately affecting older adults and postmenopausal women [13]. Unlike traditional heart failure, where the heart’s ability to contract is weakened, HFpEF is marked by stiff, less compliant ventricles that impair the heart’s ability to relax and fill during diastole, the phase when blood flows into the heart.
Patients with HFpEF often experience persistent fatigue, shortness of breath with mild exertion, fluid retention, and frequent hospitalizations. Yet despite its prevalence and impact, there are few, if any, disease-modifying therapies currently available. Today’s treatment strategies largely focus on symptom management. Clinicians may prescribe diuretics to reduce fluid buildup, medications to lower blood pressure, or recommend lifestyle changes, such as diet, exercise, and blood sugar control. While helpful, these interventions do not target the root biological causes of HFpEF.
At the cellular level, HFpEF is driven by multiple age-related changes, including:
Overactivation of the mTOR pathway, particularly mTORC1
Declining autophagy, the cell’s internal cleanup process
Mitochondrial dysfunction, which compromises energy production
Fibrosis, the accumulation of stiff, scar-like tissue within the heart
Strikingly, these same mechanisms have been observed not only in aging humans but also in animal models, from laboratory mice to the naturally aging cats in the RAPACAT studies. This convergence has led researchers to explore whether targeting mTOR with rapamycin could address the root causes of cardiac dysfunction. Rapamycin, by inhibiting mTORC1, helps restore cellular balance, shifting the heart away from maladaptive growth and toward repair. By promoting autophagy, reducing oxidative stress, and limiting fibrosis, rapamycin has the potential to make the heart more flexible and better able to fill during diastole, without affecting its pumping strength.
Evidence in humans, though still early, is promising. In a landmark study by Mannick et al. (2014), older adults given low doses of everolimus, a rapamycin derivative, showed improved immune responses, including stronger reactions to influenza vaccines [14]. Rather than suppressing immunity, the treatment appeared to recalibrate it, supporting a new framework for how rapalogs might enhance resilience in aging populations. Building on that foundation, newer clinical trials are now testing whether rapamycin can improve cardiac structure and function directly.
In feline models with naturally occurring hypertrophic cardiomyopathy, rapamycin has shown the ability to do more than treat symptoms. It reduces left ventricular wall thickness, restores autophagy, decreases fibrosis, and improves relaxation during diastole. These aren’t short-term changes; they reflect deep molecular recalibration (a biological reset) within the aging heart.
The cellular hallmarks of cardiac aging, mTOR overactivation, impaired autophagy, mitochondrial dysfunction, and fibrotic remodeling, are not isolated observations. They are deeply conserved signatures of decline, repeated across species and models. This convergence points not just to a shared vulnerability, but to a shared therapeutic opportunity.
Rapamycin, by targeting the central node of this aging network, mTORC1, has demonstrated the ability to reverse or mitigate these dysfunctions across a wide range of organisms. From yeast and mice to companion animals and early human trials, the same patterns emerge: reduced pathological growth, restored cellular cleanup, improved tissue flexibility. The consistency of these effects suggests we are not merely treating disease, but rebalancing a fundamental biological program.
As clinical research progresses, the key question is no longer whether mTOR plays a central role in age-related cardiac decline, but how best to intervene, when, at what dose, and in whom. The convergence of data from laboratory, veterinary, and early human studies builds a compelling case for further exploration. Future work must now resolve the practical challenges of implementation, but the mechanistic groundwork is clear: age-related cardiac dysfunction is a tractable target.
TAKE HOME POINTS
Hypertrophy as a Hyperfunctional Disorder. Rather than reflecting cardiac failure, hypertrophic cardiomyopathy (HCM) exemplifies pathological overfunction. In response to persistent anabolic signaling—especially via mTORC1—cardiac myocytes enlarge, stiffen, and remodel, not from degeneration, but from continued cellular growth beyond what is physiologically necessary. This aligns with the hyperfunction theory of aging, which reframes disease as an extension of normal growth pathways left unchecked.
mTORC1 as a Central Driver of Cardiac Aging. The mTORC1 signaling complex, vital for early development, becomes maladaptive in adulthood when persistently activated. It drives protein synthesis, hypertrophy, and metabolic demand, while simultaneously suppressing autophagy. In the heart, this contributes to mitochondrial dysfunction, oxidative stress, and fibrotic remodeling—hallmarks of diastolic dysfunction and HFpEF.
Autophagy as a Therapeutic Pivot. Autophagy—the process of cellular recycling—is essential for maintaining heart cell health. As mTORC1 activity increases with age, autophagy declines. This imbalance leads to the buildup of damaged mitochondria and protein aggregates, impairing cardiac energy efficiency and accelerating tissue stiffening. Rapamycin restores autophagic flux, clearing cellular debris and promoting myocardial resilience.
Feline HCM as a Translational Aging Model. Domestic cats are one of the few species to develop spontaneous, age-related HCM without genetic engineering. Their disease mirrors human cardiac aging in its natural onset, molecular drivers, and clinical trajectory, making feline HCM a uniquely powerful model for studying mTOR-driven cardiomyopathy in real-world conditions.
Rapamycin’s Role in Rebalancing Cardiac Growth. By selectively inhibiting mTORC1, rapamycin redirects cellular priorities from growth to maintenance. In aging feline hearts, this translates to reduced wall thickness, improved relaxation, and suppressed fibrosis. These effects suggest rapamycin doesn’t just slow disease—it may actively reverse maladaptive remodeling.
Cardiac Remodeling Reversed in RAPACAT. In the RAPACAT clinical trial, cats treated with rapamycin showed a 17–22% reduction in left ventricular wall thickness and improved diastolic filling—without compromising ejection fraction. Molecular data confirmed increased autophagy and reduced mTOR activity, validating that rapamycin was hitting its biological target.
mTORC2 and the Dose-Dependent Risk. While rapamycin targets mTORC1, high-dose or chronic administration can inhibit mTORC2—an essential regulator of insulin sensitivity and immune function. Disruption of mTORC2 is associated with glucose intolerance and impaired T cell responses, echoing side effects observed in transplant patients.
Intermittent Dosing to Minimize Side Effects. Weekly or thrice-weekly low-dose rapamycin protocols appear to inhibit mTORC1 while sparing mTORC2. In feline studies, this approach preserved insulin sensitivity and immune function, offering a template for how longevity-targeting interventions might be safely implemented in clinical practice.
Fibrosis as a Reversible Hallmark. Myocardial fibrosis—once thought to be irreversible—was significantly reduced in rapamycin-treated cats. Less collagen deposition and better ventricular compliance point to the possibility that even structural aging in the heart can be modulated through targeted interventions.
Aging Hearts Share a Molecular Language. Whether in humans or cats, aging hearts show the same biological signatures: mTORC1 hyperactivity, impaired autophagy, mitochondrial dysfunction, and fibrosis. Rapamycin’s ability to reverse these across species suggests that we are not simply treating symptoms—we are intervening in a conserved cellular program that governs cardiac aging itself.
Citations
Blagosklonny M. V. (2017). From rapalogs to anti-aging formula. Oncotarget, 8(22), 35492–35507. https://doi.org/10.18632/oncotarget.18033
Saxton, R. A., & Sabatini, D. M. (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell, 168(6), 960–976. https://doi.org/10.1016/j.cell.2017.02.004
Golla, M. S. G., & Shams, P. (2024, March 19). Heart failure with preserved ejection fraction (HFpEF). In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK599960/
Fox, P. R., Keene, B. W., Lamb, K., Schober, K. A., Chetboul, V., Luis Fuentes, V., Wess, G., Payne, J. R., Hogan, D. F., Motsinger-Reif, A., Häggström, J., Trehiou-Sechi, E., Fine-Ferreira, D. M., Nakamura, R. K., Lee, P. M., Singh, M. K., Ware, W. A., Abbott, J. A., Culshaw, G., Riesen, S., … Tachika Ohara, V. Y. (2018). International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL Study. Journal of Veterinary Internal Medicine, 32(3), 930–943. https://doi.org/10.1111/jvim.15122
Tawfik, D., & Rose, J. (n.d.). Rapamycin’s role as a molecular brake for cellular hyperfunction and runaway cells. Healthspan. Retrieved June 24, 2025, from https://gethealthspan.com/science/article/hyperfunction-rapamycin-mtor-driven-aging-blagosklonny
Kobak, K. A., Zarzycka, W., King, C. J., Peelor, F. F., Miller, B. F., & Chiao, Y. A. (2023). Abstract P2088: Proteostatic imbalance in heart failure with preserved ejection fraction is accompanied by mechanistic target of rapamycin complex 1 hyperactivation. Circulation Research, 133(Suppl_1). https://doi.org/10.1161/res.133.suppl_1.p2088
Kobak, K. A., Zarzycka, W., King, C. J., Borowik, A. K., Peelor, F. F., 3rd, Baehr, L. M., Leutert, M., Rodriguez-Mias, R. A., Villén, J., Bodine, S. C., Kinter, M. T., Miller, B. F., & Chiao, Y. A. (2025). Proteostatic Imbalance Drives the Pathogenesis and Age-Related Exacerbation of Heart Failure With Preserved Ejection Fraction. JACC: Basic to Translational Science, 10(4), 475–497. https://doi.org/10.1016/j.jacbts.2024.11.006
Payne, J. R., Brodbelt, D. C., & Luis Fuentes, V. (2015). Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). Journal of Veterinary Cardiology: The Official Journal of the European Society of Veterinary Cardiology, 17 Suppl 1, S244–S257. https://doi.org/10.1016/j.jvc.2015.03.008
Kittleson, M. D., & Côté, E. (2021). The Feline Cardiomyopathies: 2. Hypertrophic cardiomyopathy. Journal of Feline Medicine and Surgery, 23(11), 1028–1051. https://doi.org/10.1177/1098612X211020162
Lamming, D. W., Ye, L., Katajisto, P., Goncalves, M. D., Saitoh, M., Stevens, D. M., Davis, J. G., Salmon, A. B., Richardson, A., Ahima, R. S., Guertin, D. A., Sabatini, D. M., & Baur, J. A. (2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science, 335(6076), 1638–1643. https://doi.org/10.1126/science.1215135
Rivas, V. N., Kaplan, J. L., Kennedy, S. A., Fitzgerald, S., Crofton, A. E., Farrell, A., Grubb, L., Jauregui, C. E., Grigorean, G., Choi, E., Harris, S. P., & Stern, J. A. (2023). Multi-Omic, Histopathologic, and Clinicopathologic Effects of Once-Weekly Oral Rapamycin in a Naturally Occurring Feline Model of Hypertrophic Cardiomyopathy: A Pilot Study. Animals, 13(20), 3184. https://doi.org/10.3390/ani13203184
Kaplan, J. L., Rivas, V. N., Walker, A. L., Grubb, L., Farrell, A., Fitzgerald, S., Kennedy, S., Jauregui, C. E., Crofton, A. E., McLaughlin, C., Van Zile, R., DeFrancesco, T. C., Meurs, K. M., & Stern, J. A. (2023). Delayed-release rapamycin halts progression of left ventricular hypertrophy in subclinical feline hypertrophic cardiomyopathy: results of the RAPACAT trial. Journal of the American Veterinary Medical Association, 261(11), 1628–1637. https://doi.org/10.2460/javma.23.04.0187
Groenewegen, A., Rutten, F. H., Mosterd, A., & Hoes, A. W. (2020). Epidemiology of heart failure. European Journal of Heart Failure, 22(8), 1342–1356. https://doi.org/10.[1002/ejhf.1858
Mannick, J. B., Del Giudice, G., Lattanzi, M., Valiante, N. M., Praestgaard, J., Huang, B., Lonetto, M. A., Maecker, H. T., Kovarik, J., Carson, S., Glass, D. J., & Klickstein, L. B. (2014). mTOR inhibition improves immune function in the elderly. Science Translational Medicine, 6(268), 268ra179. https://doi.org/10.1126/scitranslmed.3009892
Latest Longevity Research Straight to your Inbox
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.
_Madeline_Spanier_Photos_ID7424%20(1).jpg?u=https%3A%2F%2Fimages.ctfassets.net%2Fzvzqa1d1gh0f%2F2OqcwI9wXXYvDjaPxL2RHx%2F704d7e24be14806edd4c42c57649e84d%2FDTS_MISC_3__Maddie_Spanier__Madeline_Spanier_Photos_ID7424__1_.jpg&a=w%3D223%26h%3D110%26fm%3Djpg%26q%3D75&cd=2025-07-12T16%3A39%3A42.347Z)